what happens to atom in a chemical reaction
Introduction to chemical reactions
Spring to
Primal points
- Chemic reactions make new chemicals.
- Atoms are rearranged during a chemic reaction, just the number of atoms does not change.
- Evidence of chemical reactions includes a large temperature alter, bubbles, or a colour modify.
- Chemic reactions can be represented using equations.
Video
Watch this video to observe out what happens to atoms in a chemical reaction.
Chemical reactions.
Compounds are chemical substances that contain more than i element. They're created during a chemical reaction where atoms are rearranged into new chemical compound molecules. For example, if carbon atoms react with oxygen atoms they form carbon dioxide molecules. Carbon dioxide is present in the temper and contributes to global warming.
We write this reaction equally a discussion equation and equally the chemical formula, CO₂.
This tells us there are two oxygen atoms for every single carbon atom.
Bear witness for chemical reactions
Evidence for a chemical reaction can include whatsoever of the post-obit:
- Bubbles – Many chemic reactions you meet in the science lab brand a chemic which is a gas, so yous meet bubbling.
- A colour change – If the new chemicals are a different colour from the original chemicals, there will be a colour modify.
- A large energy modify – Many chemic reactions give off lots of energy, like burning, and a few absorb energy, then they feel cold.
A new substance must be produced for a chemic reaction to accept place.
The water inside a kettle has bubbles just that's just considering it is boiling. Bubbles of steam class in boiling water. These bubbles do non point a new substance has been formed because, when cooled, the steam condenses back into liquid water. Boiling is a , not a chemic reaction.
How are chemical reactions used?
-
Lots of chemical reactions are used to brand useful new chemicals, like plastics and medicines.
-
Many useful chemical reactions involve burning fuels to release energy. These reactions oestrus our homes, power our cars and generate lots of the electricity that we use.
-
Cooking uses chemical reactions to make foods which are condom and tasty to eat.
Video
Picket this video about how a chef uses in their kitchen to make honeycomb.
Every mean solar day in my kitchen there's reactions happening all effectually me all the time, from me coming in lighting the stove, baking, cooking.
We're making honeycomb, which the kids absolutely love and we do this in the kids cookery classes.
First of all we demand to add the beloved...
Adjacent nosotros are going to add the glucose but I am actually going to use some gilt syrup.
Some sugar. A little bit of water.
And finally nosotros add the bicarbonate of soda and this is where the magic happens.
And you go this chemical reaction and it looks like lava.
So we're gonna get out that at that place for about an hour and so once it hardens we boom it into pieces and it's fix to eat.
Making CO₂ from vinegar and baking soda
If you lot mix vinegar and baking soda, a reaction volition occur, where lots of bubbles of carbon dioxide gas grade.
You tin can do this reaction at home just make sure you don't become any of the chemicals in your eyes. Ethanoic acid is the chemic name for vinegar. Sodium bicarbonate is also known equally baking soda.
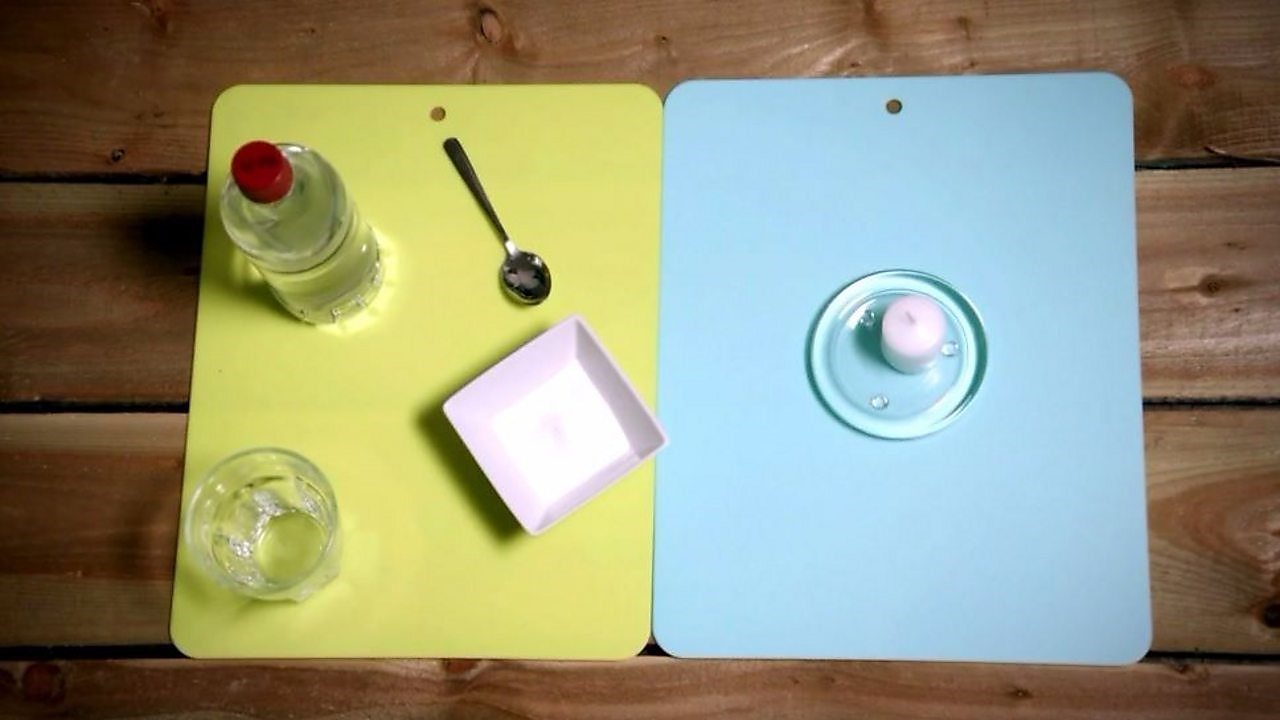
WHAT You lot NEED: vinegar, bicarbonate of soda, a pocket-sized glass, a teaspoon and a candle.

Footstep ane: Light the candle. REMEMBER: lit candles are a fire adventure, so brand sure you take an adult with you!

STEP 2: Pour 100 millilitres of vinegar into a large glass. The vinegar contains ethanoic acrid.

STEP iii: Add a small teaspoon of bicarbonate of soda. The scientific name for this is sodium bicarbonate. The mixture should get-go to foam as the sodium bicarbonate reacts with the ethanoic acid and produces bubbles of carbon dioxide gas.

Stride 4: Cascade the carbon dioxide onto the flame. The CO₂ gas is heavier than air so pours down onto the burning candle wick. It displaces the oxygen fuelling the flame and puts it out!
A particle model of a chemical reaction
In this animation, watch how sodium bicarbonate reacts with ethanoic acrid.
Notice that…
- there are the aforementioned number of particles at the beginning of the reaction as there are after information technology
- the two reactants have broken down and reformed as three products
- sodium bicarbonate + ethanoic acrid → sodium ethanoate + water + carbon dioxide
Chemic reactions and physical changes
- Physical changes, such as melting, boiling and dissolving, do not make new chemicals. They are usually easy to reverse.
- In a chemical reaction, chemical bonds between atoms are broken and made, and then the atoms get rearranged into new substances.
- The simplest kind of chemical reactions involve 2 reacting together to make a .
Representing a chemical reaction
Run into what happens in the chemical reaction when iron and sulfur are heated. A sulfide is a chemical compound containing the element sulfur.
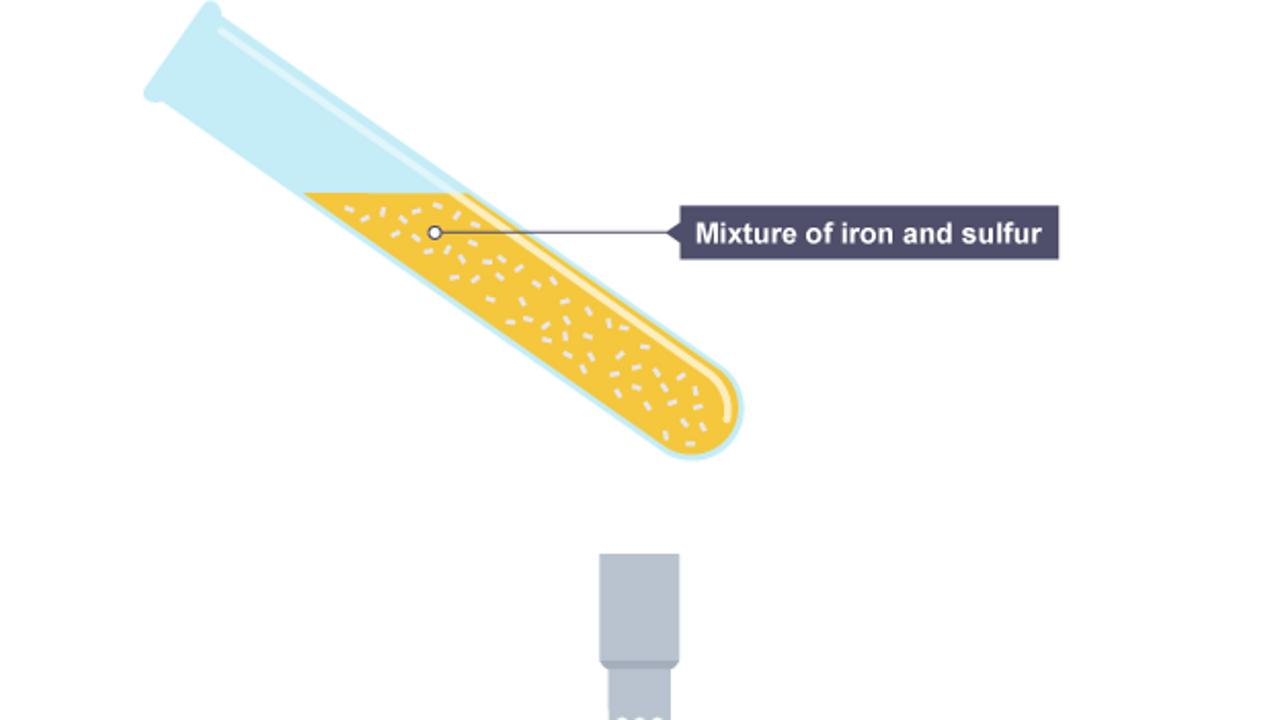
The test tube is partly filled with a mixture of atomic number 26 and sulfur.
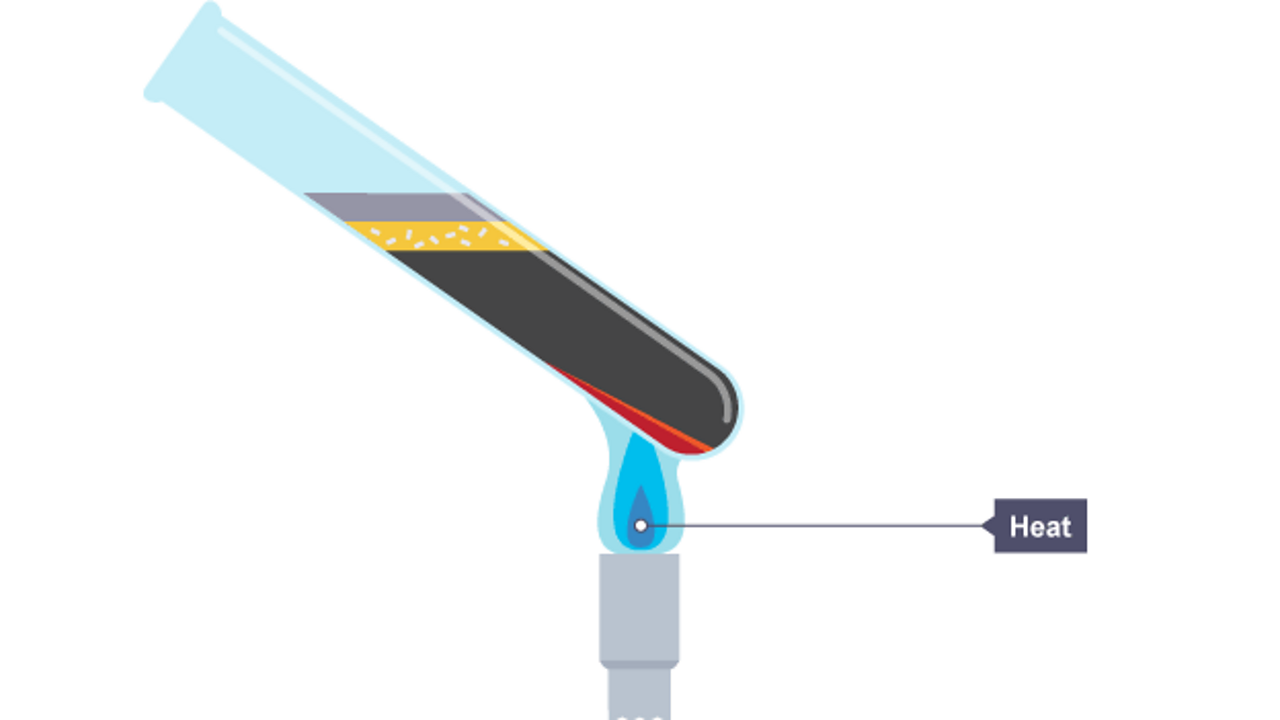
The mixture is heated strongly using a bunsen burner.
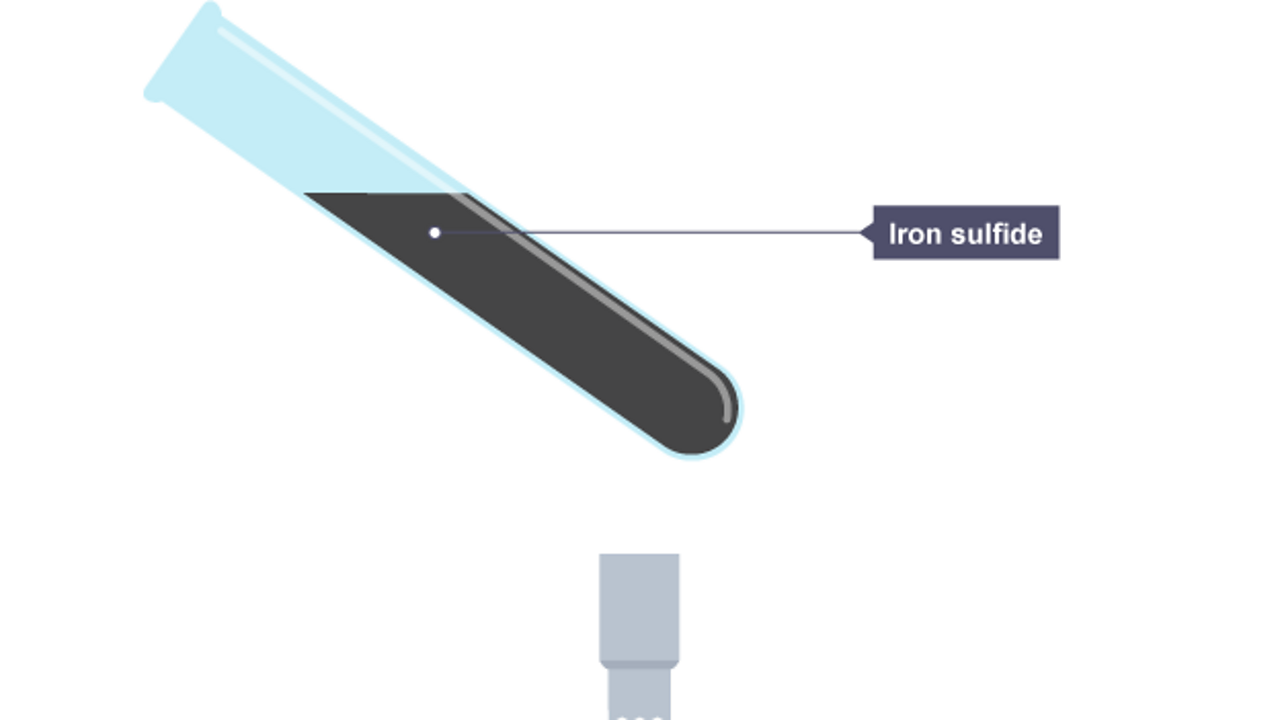
The test tube at present contains iron sulfide.
Working scientifically
Types of data
If you carry out an investigation you volition record evidence of whatsoever changes. The evidence you tape is chosen data and it may exist quantitative or qualitative.
Quantitative data
Quantitative data tin can be counted, measured, and expressed using numbers.
Quantitative data can exist discrete (counted) or continuous (measured).
For example, if a reaction releases a lot of free energy we tin can use a thermometer and measure the temperature rise in degrees Celsius (C).
Qualitative data (words)
Qualitative data describes qualities or characteristics which are often observable.
For example, "the colour changed from white to blue".
Yous must always state what the colour was and what it changed also.
Data recorded in scientific experiments tin exist quantitative or qualitative.
Test your knowledge
Source: https://www.bbc.co.uk/bitesize/topics/zypsgk7/articles/zwxhk2p
Post a Comment for "what happens to atom in a chemical reaction"